Powder: -20°C for 3 years | In solvent: -80°C for 1 year
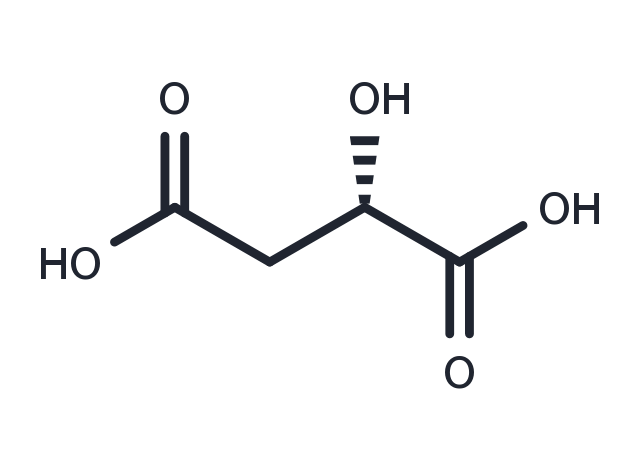
(S)-Malic acid ((S)-2-Hydroxysuccinic acid) is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. Apples contain malic acid, which contributes to the sourness of a green apple. Malic acid can make a wine taste tart, although the amount decreases with increasing fruit ripeness. (wikipedia). In its ionized form malic acid is called malate. Malate is an intermediate of the TCA cycle along with fumarate. It can also be formed from pyruvate as one of the anaplerotic reactions. In humans, malic acid is both derived from food sources and synthesized in the body through the citric acid cycle or Krebs cycle which takes place in the mitochondria. Malate's importance to the production of energy in the body during both aerobic and anaerobic conditions is well established. Under aerobic conditions, the oxidation of malate to oxaloacetate provides reducing equivalents to the mitochondria through the malate-aspartate redox shuttle. During anaerobic conditions, where a buildup of excess of reducing equivalents inhibits glycolysis, malic acid's simultaneous reduction to succinate and oxidation to oxaloacetate is capable of removing the accumulating reducing equivalents. This allows malic acid to reverse hypoxia's inhibition of glycolysis and energy production. In studies on rats it has been found that only tissue malate is depleted following exhaustive physical activity. Other key metabolites from the citric acid cycle needed for energy production were found to be unchanged. Because of this, a deficiency of malic acid has been hypothesized to be a major cause of physical exhaustion. Notably, the administration of malic acid to rats has been shown to elevate mitochondrial malate and increase mitochondrial respiration and energy production.
| パッケージサイズ | 在庫状況 | 単価(税別) | |||
|---|---|---|---|---|---|
| サンプルについてお問い合わせ | |||||
| 5 g | 在庫あり | ¥ 7,000 | |||
| 10 g | 在庫あり | ¥ 8,000 | |||
| 1 mL * 10 mM (in DMSO) | 在庫あり | ¥ 7,000 | |||
| 説明 | (S)-Malic acid ((S)-2-Hydroxysuccinic acid) is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. Apples contain malic acid, which contributes to the sourness of a green apple. Malic acid can make a wine taste tart, although the amount decreases with increasing fruit ripeness. (wikipedia). In its ionized form malic acid is called malate. Malate is an intermediate of the TCA cycle along with fumarate. It can also be formed from pyruvate as one of the anaplerotic reactions. In humans, malic acid is both derived from food sources and synthesized in the body through the citric acid cycle or Krebs cycle which takes place in the mitochondria. Malate's importance to the production of energy in the body during both aerobic and anaerobic conditions is well established. Under aerobic conditions, the oxidation of malate to oxaloacetate provides reducing equivalents to the mitochondria through the malate-aspartate redox shuttle. During anaerobic conditions, where a buildup of excess of reducing equivalents inhibits glycolysis, malic acid's simultaneous reduction to succinate and oxidation to oxaloacetate is capable of removing the accumulating reducing equivalents. This allows malic acid to reverse hypoxia's inhibition of glycolysis and energy production. In studies on rats it has been found that only tissue malate is depleted following exhaustive physical activity. Other key metabolites from the citric acid cycle needed for energy production were found to be unchanged. Because of this, a deficiency of malic acid has been hypothesized to be a major cause of physical exhaustion. Notably, the administration of malic acid to rats has been shown to elevate mitochondrial malate and increase mitochondrial respiration and energy production. |
| In vitro | Research indicates that ME plays a crucial role in the metabolism of (S)-2-Hydroxysuccinic acid (L-malic acid) by L. casei. Removing either the histidine kinase gene or the response regulator gene of the TC system eliminates the organism's capacity to metabolize (S)-2-Hydroxysuccinic acid. This outcome underscores the TC system's pivotal regulatory role and its necessity for ME expression to facilitate (S)-2-Hydroxysuccinic acid utilization. Additionally, transcriptional analysis reveals that the presence of (S)-2-Hydroxysuccinic acid triggers maeE expression and inhibits it in the presence of glucose. Conversely, TC system expression is upregulated by (S)-2-Hydroxysuccinic acid without being inhibited by glucose, highlighting distinct regulatory mechanisms for ME and the TC system in response to (S)-2-Hydroxysuccinic acid and glucose. |
| 別名 | L-(-)-Malic acid, (S)-(-)-HYDROXYSUCCINIC ACID, (S)-2-Hydroxysuccinic acid |
| 分子量 | 134.09 |
| 分子式 | C4H6O5 |
| CAS No. | 97-67-6 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
DMSO: 27.5 mg/mL (205.09 mM)
You can also refer to dose conversion for different animals. 詳細
bottom
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc.
(S)-Malic acid 97-67-6 Metabolism Others Endogenous Metabolite HYDROXYSUCCINIC ACID L-(-)-Malic acid inhibit (S)-(-)-HYDROXYSUCCINIC ACID Inhibitor Malic acid (S)-Hydroxybutanedioic acid (S)-E 296 (S) Malic acid 2-Hydroxysuccinic acid (S)-2-Hydroxysuccinic acid L-Malic acid (S)Malic acid inhibitor
