Powder: -20°C for 3 years | In solvent: -80°C for 1 year
Infigratinib (NVP-BGJ398) (BGJ398) is an orally bioavailable pan FGFR inhibitor (IC50: 0.9/1.4/1 nM for FGFR1/2/3), >40-fold selective for FGFR versus FGFR4 and VEGFR2.
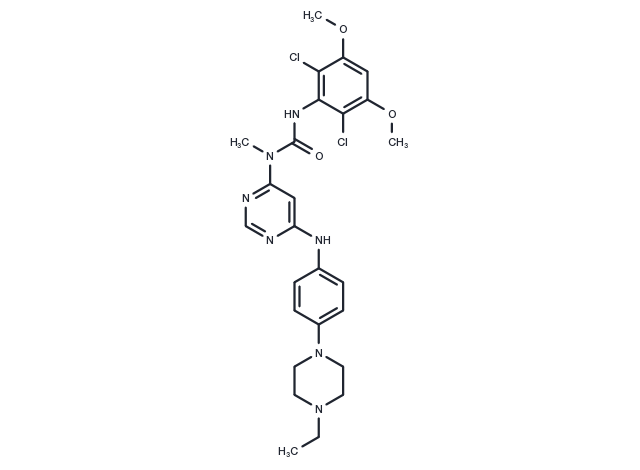
| 説明 | Infigratinib (NVP-BGJ398) (BGJ398) is an orally bioavailable pan FGFR inhibitor (IC50: 0.9/1.4/1 nM for FGFR1/2/3), >40-fold selective for FGFR versus FGFR4 and VEGFR2. |
| ターゲット&IC50 | FGFR4:60 nM (cell free), FGFR3:1.0 nM (cell free), FGFR3 (K650E):4.9 nM (cell free), FGFR1:0.2 nM (cell free), FGFR2:1.4 nM (cell free) |
| In vitro | Infigratinib (NVP-BGJ398) inhibited FGFR1/2/3 (IC50: 1 nM), FGFR3-K650E (IC50: 4.9 nM), and FGFR4 (IC50: 60 nM). NVP-BGJ398 inhibited the proliferation of the FGFR1-, FGFR2-, and FGFR3-dependent BaF3 cells with IC50 values which were in the low nanomolar range and comparable to those observed for the inhibition of the receptors kinase activity in the enzymatic assay [1]. Among the 35 cell lines selected from the high-throughput assays, 28 were confirmed as sensitive to NVP-BGJ398 (IC50s: 0.001 to 500 nmol/L). Collectively, among the 541 (517 + 24) cell lines from the CCLE subjected to viability testing, 5.9% were found to be sensitive to NVP-BGJ398 [2]. Cell lines with activating FGFR2 mutations (S252W, N550K) were more sensitive to NVP-BGJ398 when compared with their FGFR2 wild-type counterparts. NVP-BGJ398 was potent at inhibiting cell growth of FGFR2-mutant endometrial cancer cells [3]. |
| In vivo | When tested in this orthotopic xenograft bladder cancer model, NVP-BGJ398 (10 and 30 mg/kg) induced tumor growth inhibition and stasis after oral administration for 12 consecutive days. The monophosphate salt of 1h was orally administered to juvenile, immunocompromised, female Rowett rats for 20 consecutive days (20 administrations) at the doses of 5, 10, and 15 mg/kg/qd (free base equivalents). Nearly complete tumor stasis was achieved at the lowest dose while no overt toxicity was observed. The doses of 10 and 15 mg/kg provided tumor regression which was accompanied by a dose-dependent decrease of body weight [1]. NVP-BGJ398 (30 mg/kg, p.o.) significantly delayed the growth of FGFR2-mutated endometrial cancer xenograft tumors. NVP-BGJ398 had no in vivo inhibitory effects in the long-term study using the FGFR2 wild-type endometrial cancer cell line SNGM [2]. |
| キナーゼ試験 | The enzymatic kinase activity is assessed by measuring the phosphorylation of a synthetic substrate by the purified GST-fusion FGFR3-K650E kinase domain, in the presence of radiolabeled ATP. Enzyme activities are measured by mixing 10 μL of a 3-fold concentrated NVP-BGJ398 solution or control with 10 μL of the corresponding substrate mixture (peptidic substrate, ATP and [γ33P]ATP). The reactions are initiated by addition of 10 μL of a 3-fold concentrated solution of the enzyme in assay buffer. The final concentrations of the assay components are as following: 10 ng of GST-FGFR3-K650E, 20 mM Tris-HCl, pH 7.5, 3 mM MnCl2, 3 mM MgCl2, 1 mM DTT, 250 μg/mL PEG 20000, 2 μg/mL poly(EY) 4:1, 1% DMSO and 0.5 μM ATP (γ-[33P]-ATP 0.1 μCi). The assay is carried out according to the filter binding (FB) method in 96-well plates at room temperature for 10 min in a final volume of 30 μL including the components as indicated above. The enzymatic reactions are stopped by the addition of 20 μL of 125 mM EDTA, and the incorporation of 33P into the polypeptidic substrates is quantified as following: 30 μL of the stopped reaction mixture are transferred onto Immobilon-PVDF membranes previously soaked for 5 min with methanol, rinsed with water, soaked for 5 min with 0.5% H3PO4, and mounted on vacuum manifold with disconnected vacuum source. After spotting, vacuum is connected, and each well rinsed with 0.5% H3PO4 (200 μL). Free membranes are removed and washed four times on a shaker with 1% H3PO4 and once with ethanol. Membranes are dried and overlaid with addition of 10 μL/well of scintillation fluid. The plates are eventually sealed and counted in a microplate scintillation counter. IC50 values are calculated by linear regression analysis of the percentage inhibition of NVP-BGJ398 [1]. |
| 細胞研究 | Murine BaF3 cell lines, whose proliferation and survival has been rendered IL-3-independent by stable transduction with tyrosine kinases activated either by mutation or fusion with a dimerizing partner, were cultured in RPMI-1640 media supplemented with 10% FBS, 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, and Pen/Strep. Cells were passaged twice weekly. Compound-mediated inhibition of BaF3 cell proliferation and viability was assessed using a Luciferase bioluminescent assay. Exponentially growing BaF3 or BaF3 Tel-TK cells were seeded into 384-well plates (4250 cells/well) at 50 μL/well using a μFill liquid dispenser in fresh medium. NVP-BGJ398 was serially diluted in DMSO and arrayed in a polypropylene 384-well plate. Then 50 nL of compound was transferred into the plates containing the cells by using the pintool transfer device, and the plates incubated at 37 ℃ (5% CO2) for 48 h. Then 25 μL of Bright-Glo were added, and luminescence was quantified using an Analyst-GT. Custom curve-fitting software was used to produce a logistic fit of percent cell viability as a function of the logarithm of inhibitor concentration. The IC50 value was determined as the concentration of compound needed to reduce cell viability to 50% of a DMSO control [1]. |
| 動物実験 | We used FGFR2-mutated MFE296, AN3CA, and FGFR2 wild-type SNGM and HEC1A endometrial cancer cells, which spontaneously formed xenografts in athymic mice for our in vivo studies. Mice were maintained and handled under aseptic conditions, and animals were allowed access to food and water ad libitum. Female athymic mice (20.0–30.0 g) aged 4 to 6 weeks from an outbred strain (CD1 nu/nu) were injected subcutaneously (2 × 10^7 cells per mouse) in the flank. AN3CA cells were suspended in Matrigel and DMEM (volume 1:1). A period of 7 days elapsed to allow the formation of tumor nodules (mean xenograft volume = 105 ± 5.6 mm^3). Mice were then stratified into treatment groups with one tumor per mouse on the basis of their weight and tumor volume at the start of the experiment, such that the starting weight and tumor volume in each group were uniform. Mice (10/group) were treated via oral gavage of (i) vehicle control (5 mmol/L sodium citrate and 1 μL 6N HCL/mL), (ii) NVP-BGJ398 30 mg/kg (6 mg in 0.5 mL PEG300 and 0.5 mL acetic acid/acetate buffer, pH 4.68), (iii) dovitinib 30 mg/kg (1 mg in 1 mL of 5 mmol/L sodium citrate and 1 μL 6N HCL/mL), and (iv) dovitinib 50 mg/kg (1 mg in 1 mL of 5 mmol/L sodium citrate and 1 μL 6N HCL/mL). Treatment was continuous on a daily basis. Tumors were monitored by serial micrometer measurements made by a single observer with tumor volumes calculated length × width × depth. Differences in xenograft volume between groups were analyzed by Student t test of the tumor volume data [3]. |
| 別名 | BGJ-398, NVP-BGJ398 |
| 分子量 | 560.48 |
| 分子式 | C26H31Cl2N7O3 |
| CAS No. | 872511-34-7 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
DMSO: 5.61 mg/mL (10 mM), Sonication is recommended.
You can also refer to dose conversion for different animals. 詳細
bottom
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc.
Infigratinib 872511-34-7 Angiogenesis Apoptosis Tyrosine Kinase/Adaptors FGFR Fibroblast growth factor receptor BGJ 398 BGJ-398 NVP-BGJ398 inhibit Inhibitor NVP-BGJ-398 NVP-BGJ 398 BGJ398 inhibitor
